Introduction
Pancreatic neuroendocrine tumors (PanNETs) are uncommon tumors responsible for up to 2% of clinically detected pancreatic tumors. Although anatomic imaging modalities including the US, multidetector CT, and MRI are helpful for the initial diagnosis by revealing typical radiologic appearances of PanNET, several other lesions can mimic PanNET, especially when the tumor is exophytic or pedunculated. Herein, an exophytic PanNET that complicated the radiologic diagnosis is presented.
Case Report
A 51-year-old female patient was referred to our hospital for the evaluation of a pancreatic mass incidentally detected during a screening US. The patient was asymptomatic, and physical examination showed no abnormality. Routine laboratory tests and the level of tumor markers were all within the normal range, including the carbohydrate antigen 19-9 of 14 U/mL.
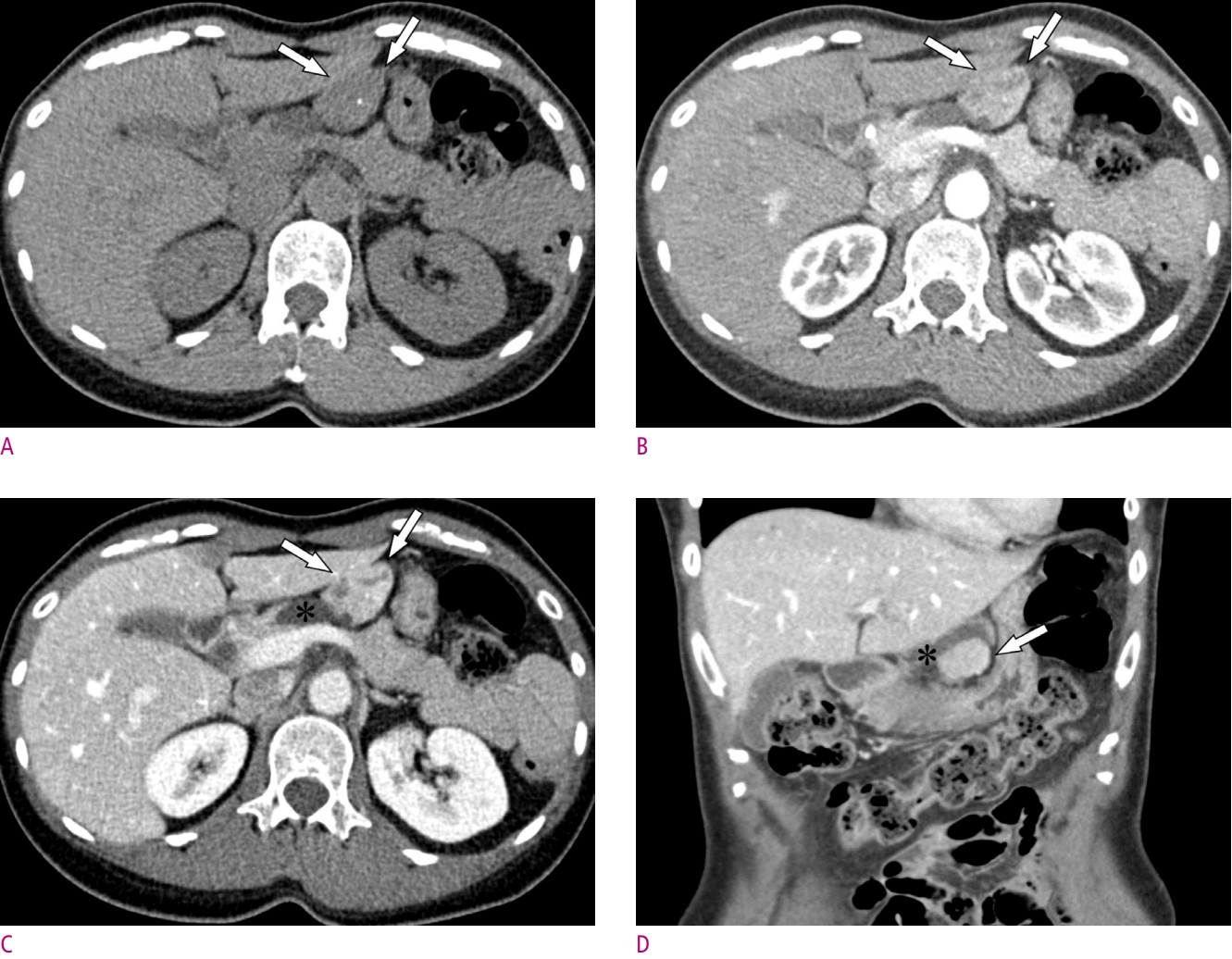
Multiphasic CT showed a 3.5-cm sized well-defined enhancing mass (arrows) on the peripancreatic area, enclosed by the pancreas, stomach, and the left lateral section of the liver (Fig. 1). On non-contrast scan (Fig. 1A), a homogeneous mass with a tiny calcification was appreciated. In axial pancreatic (Fig. 1B) and portal venous (Fig. 1C) phases, the mass enhanced well and had internal cystic components. At the posteromedial aspect of the mass, an elongated-shaped cystic lesion that abutted the anterior surface of the pancreas was attached to the mass (asterisks). The coronal portal venous phase (Fig. 1D) showed the cystic lesion surrounding the posterior surface of the mass. No enlarged lymph nodes were found in the abdomen and pelvis.
On axial (Fig. 2A) and coronal T2-weighted images (Fig. 2B, C), the mass (arrows) showed heterogeneously hyperintense T2 signal with tiny cystic components. An elongated-shaped cystic lesion (asterisks) attached to the pancreas and the mass was also well appreciated. Dynamic enhancement study using extracellular gadolinium-based contrast media (Fig. 2D-F) revealed heterogeneously enhancing mass abutting the liver. There was mild diffusion restriction (Fig. 2G, H).
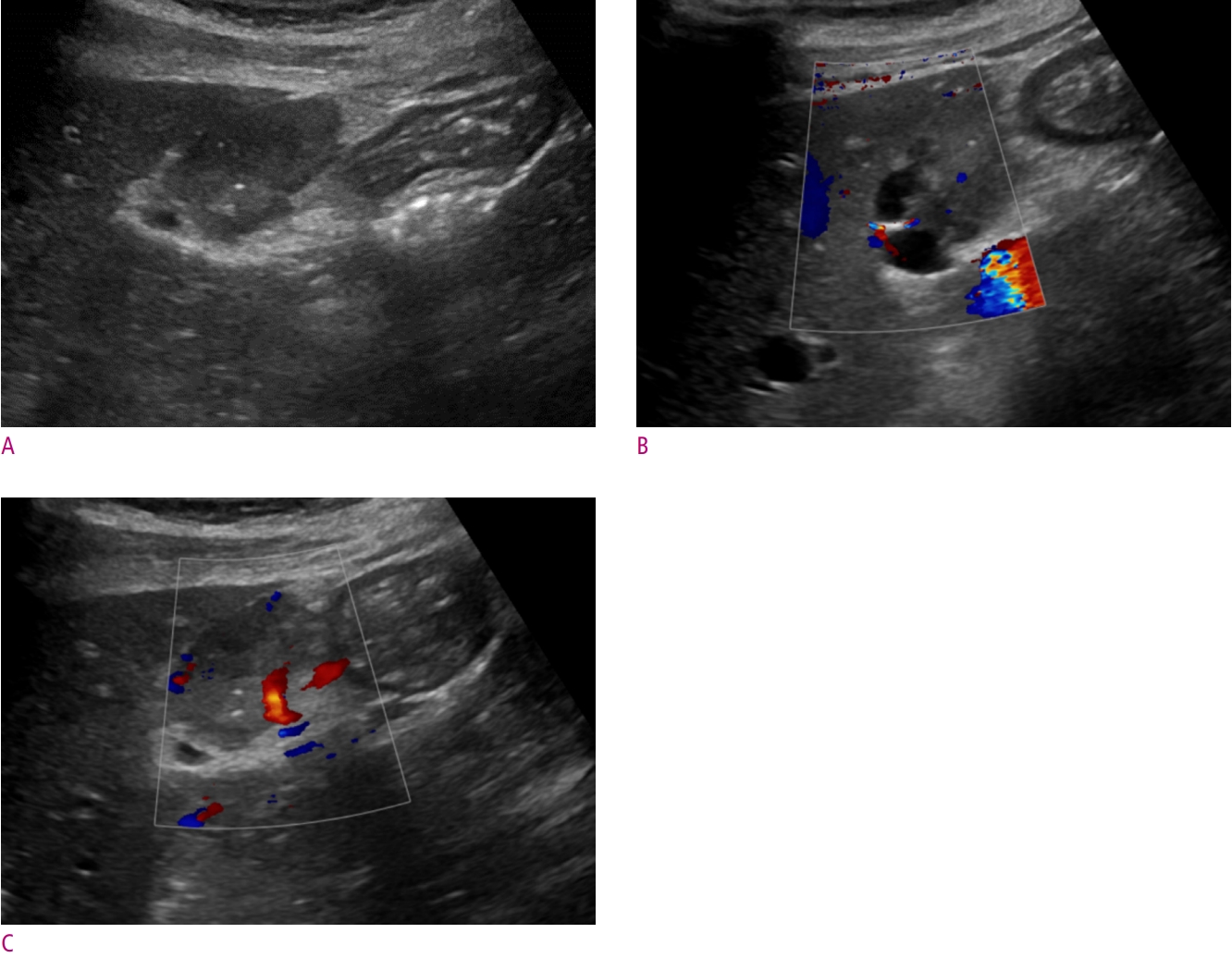
The mass was predominantly solid and contained cystic components based on these findings. Thought to have originated from the liver or the mesentery, preliminary diagnosis of benign liver mass such as adenoma or atypical hemangioma was given along with neurogenic tumor from the mesentery. For the pathologic diagnosis, a percutaneous biopsy was planned. The US during the biopsy (Fig. 3A) revealed a well-circumscribed and heterogeneously echogenic mass abutting the liver and stomach. The anechoic cystic component was evident at the periphery of the mass (Fig. 3B). Color Doppler showed prominent tumor vessels (Fig. 3C). The histopathologic result of the biopsy indicated a neuroendocrine tumor, probably grade 1.
Still unsure of the origin of the tumor, diagnostic and therapeutic laparoscopy was performed. The mass was located at the lesser sac on laparoscopic exploration and was easily separated from the liver and stomach. The elongated cystic component of the mass was traced back to identify that the mass originated from the pancreas's body. Because only thin pancreatic parenchyma would remain after the tumor enucleation, distal pancreatectomy was done.
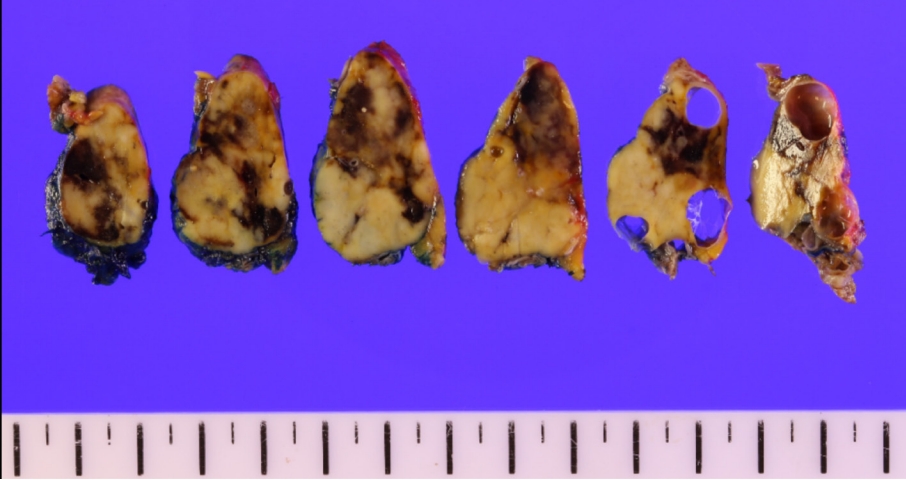
Histopathologic examination of the resected specimen revealed a 3.7 cm well-differentiated PanNET (Fig. 4) with a mitotic count of 1/10 HPF and Ki-67 proliferation rate of 1%, indicating a grade 1 tumor. Because normal pancreatic parenchyma was discovered at the periphery of the tumor, the origin was confirmed to be the pancreas. The elongated cystic lesion between the tumor and the pancreas had lined by an epithelium, suggesting a retention cyst.
Discussion
PanNETs are rare tumors responsible for up to 2% of all clinically detected pancreatic tumors. They arise from precursor cells in the pancreatic ductal epithelium with neuroendocrine differentiation [1]. The 2017 WHO classification system is the most updated classification of the pancreatic neuroendocrine neoplasm, which accounts for both the degree of cellular differentiation and cellular proliferation [1, 2]. PanNETs are well-differentiated tumors, while pancreatic neuroendocrine carcinomas are poorly differentiated tumors that consist of atypical cells with substantial necrosis. Within the PanNETs, mitotic counts and Ki-67 proliferation rate dictate tumor grade, ranging from 1 to 3.
On cross-sectional images, PanNETs are typically hyperenhancing during the early phase and remain hyperdense during the portal venous and delayed phases. Smaller tumors are homogeneous, but larger lesions can be heterogeneous owing to cystic degeneration, necrosis, and calcification. On MRI, PanNETs are usually hypointense on T1-weighted images and are hyperintense on T2-weighted images. Diffusion-weighted images increase the sensitivity for detecting PanNETs, as they often show restricted diffusion [3, 4].
Meanwhile, several atypical imaging features have been described. As high as 49% of PanNETs are hypovascular, mimicking pancreatic ductal adenocarcinomas [5]. Other unusual features include concurrent main pancreatic duct dilatation, intraductal location of the tumor, and tumor thrombus formation in peripancreatic vessels [4]. An exophytic or pedunculated PanNET is also an atypical finding, such as in the current case [6, 7]. Although the exact prevalence of the exophytic PanNET has not been reported, given that enucleation is endorsed as a surgical treatment option for exophytic PanNET, it might not be highly uncommon in clinical practice [8, 9]. In such cases, ambiguous tumor origin demands differential diagnoses, including peripancreatic gastrointestinal stromal tumor, peripancreatic paraganglioma, solitary fibrous tumor, and even lymph node [7]. The most helpful clue for an accurate radiologic diagnosis is the origin of the tumor. In the current case, the gastric subepithelial tumor was ruled out by observing clearly maintained fat plane between the tumor and the stomach on the multiplanar reformatted CT images. On the other hand, the possibility of an exophytic liver mass could not be excluded because of the diminished fat plane and negative sliding sign during the real-time US. On the retrospective review, realizing that the elongated cystic lesion was attached to both the solid tumor and pancreas could have helped the diagnosis.
In conclusion, acknowledging exophytic PanNET as a differential diagnosis of various peripancreatic masses can help in making the correct diagnosis for unusual peripancreatic lesions.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print